 Immunotherapy has sparked new hope for oncology due to its remarkable ability to induce a durable
Immunotherapy has sparked new hope for oncology due to its remarkable ability to induce a durable
response in patients with metastatic cancer. However, a large fraction of patients do not respond to
immunotherapy. The immune response against tumor cells is driven by neoantigen presentation. Yet, most
tumors harbor a low mutational burden, limiting their targetable neo-peptidomic space. We propose to
address the urgent need to expand the search for neoantigens, by focusing on neopeptides derived from
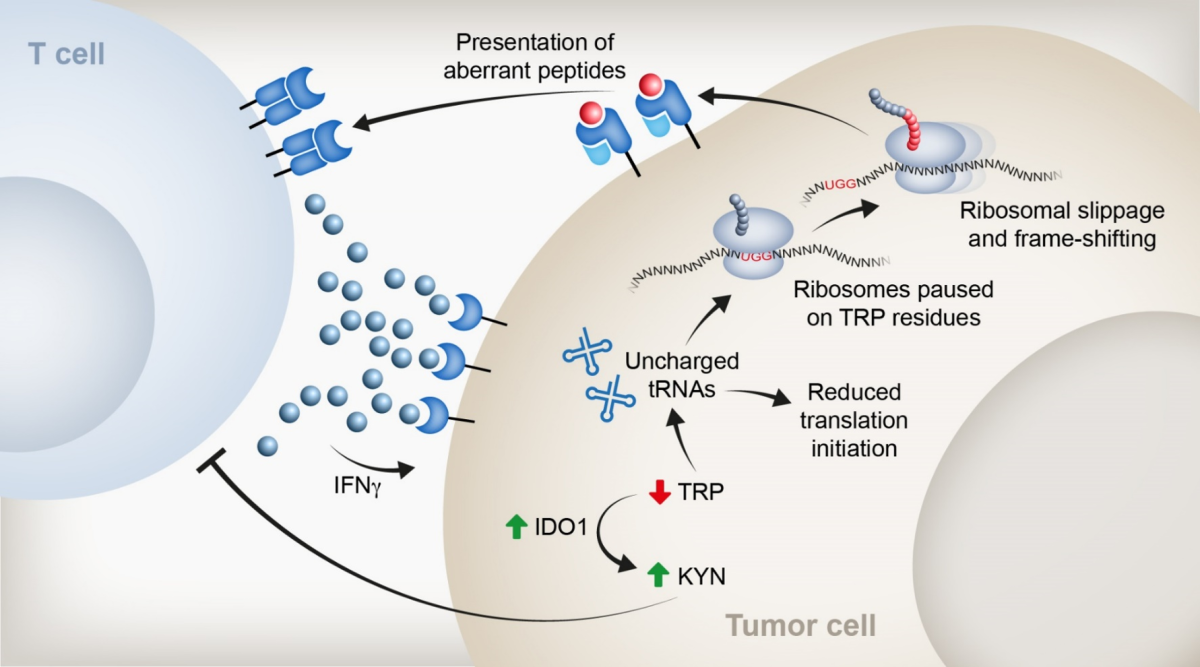
cancer-specific translation aberrations, an avenue we recently pioneered. We demonstrated that Tryptophan
depletion evokes protein synthesis events that lead to the presentation of immunogenic aberrant peptides.
Moreover, we found an additional class of tumor-presented antigens that are derived from intra-tumor
bacteria that could be used as potential immunotherapy targets.
In this proposal, we suggest utilizing bacteria to deplete tryptophan in the tumor microenvironment by
engineering bacteria that colonize melanoma tumors to overexpress tryptophan-depleting enzymes. We will
assess whether the engineered bacteria induce aberrant peptide presentation by mouse melanoma cells. We
will test in vivo whether the modified bacteria can reach and colonize tumors and cause localized tryptophan
depletion. We will further test how this depletion affects tumor growth and response to checkpoint
inhibitors. Lastly, we will identify presented aberrant peptides in vivo, test the peptides' immunogenicity,
and design mRNA vaccines targeting these peptides. The proposed studies have significant translational
implications, with the potential to identify novel strategies to enhance immunotherapy modalities applicable
to a large number of patients with various tumor mutational burdens.
Grant type:
Grant scientist:
Yardena Samuels
Grant year:
2024

