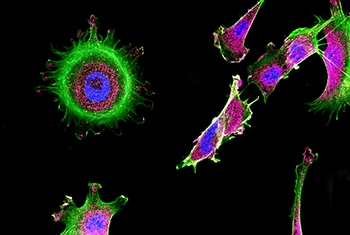
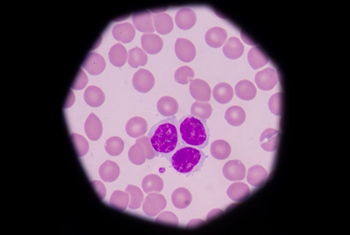
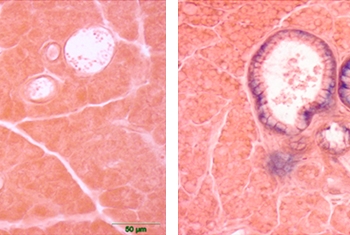
Extensive studies have established a causal link between smoking and cancer. While the danger of smoking is now well known, the general public is less aware of cancer-related risk factors stemming from air pollution. This is one area of research being pursued by Prof. Yinon Rudich of the Department of Earth and Planetary Sciences.
An expert on the physical and optical properties of aerosols—the airborne dust that contributes to the onset of cancer as well as other disease conditions—Prof. Rudich has demonstrated how exposure to suspended urban dust can reduce the body’s natural antioxidant response and increase lung cancer risk. His research is clarifying how such exposure alters gene transcription in specific genetic networks, thus identifying “biomarkers” that can be used to quantify cancer risk. As such, his work is clarifying the factors that will drive the creation of more informed environmental policy—and better health—in the future.
Read More about Identifying risk factors